Introduction
COVID-19, caused by the SARS-CoV-2 virus, has become a global health crisis since its emergence in late 2019. The severity of COVID-19 can vary widely among patients, ranging from asymptomatic or mild cases to severe pneumonia, multi-organ failure, and even death. Understanding the factors that contribute to the severity of this disease is crucial for the development of effective treatments and diagnostic tools. One such factor is the immune response, particularly the role of immunoglobulins such as IgM. Recent research has shed light on the significant role that IgM N-glycosylation plays in COVID-19 severity and complement system activation, providing valuable insights into the underlying mechanisms of the disease.
This article explores the findings from a study conducted by Mary Ann Comunale’s team at Drexel University, published in Nature Communications. Their research investigates how alterations in IgM N-glycosylation are correlated with the severity of COVID-19 and the rate of complement deposition, offering new potential biomarkers for disease severity and insights into the immune response to the virus.
The Immunological Mechanisms in COVID-19
COVID-19 severity is often linked to the body’s immune response to the SARS-CoV-2 virus. Upon infection, the immune system activates a series of defense mechanisms to recognize and eliminate the virus. One important component of the immune system is immunoglobulin M (IgM), which is produced early during an infection and plays a crucial role in pathogen recognition and neutralization.
IgM is a glycoprotein, meaning it contains sugar molecules attached to its protein backbone, a process known as glycosylation. The modification of IgM through N-glycosylation, in particular, has been shown to influence its functionality and its ability to engage with other immune system components, such as the complement system.
The complement system is a part of the immune response that enhances the ability of antibodies and phagocytes to clear pathogens from the body. The activity of the complement system is often measured by the rate of complement deposition, which can provide valuable information on the immune system’s activity during an infection. Understanding how IgM N-glycosylation affects the immune response in COVID-19 patients could offer new ways to assess disease severity and predict patient outcomes.
Altered IgM N-Glycosylation and COVID-19 Severity
In their study, Comunale’s team focused on how changes in the N-glycosylation patterns of IgM correlate with the severity of COVID-19. N-glycosylation refers to the attachment of sugar molecules to the nitrogen atom in the side chain of asparagine residues within the IgM protein. This modification plays a critical role in the stability, structure, and function of IgM.
One of the key findings of the study is that certain alterations in IgM N-glycosylation are strongly associated with the severity of COVID-19. Specifically, the presence of disialylation (addition of two sialic acid residues) and mannose modification on IgM were found to be significantly linked to more severe disease outcomes. These modifications seem to affect the ability of IgM to perform its immunological functions, which could influence the progression of COVID-19.
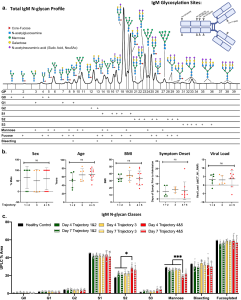
Fig.1 IgM N-glycosylation analysis reveals differences in COVID-19 patients stratified by trajectory.1
This discovery is particularly noteworthy because it highlights the potential of IgM N-glycosylation as a biomarker for COVID-19 severity. The study suggests that examining the glycosylation patterns of IgM could provide valuable insights into a patient’s immune response and the potential for severe disease.
The Role of Golgi Glycosyltransferases in IgM N-Glycosylation
The researchers also investigated the molecular mechanisms behind the alterations in IgM N-glycosylation. They found that the expression of Golgi glycosyltransferases—enzymes responsible for adding sugar molecules to proteins—was closely associated with changes in IgM N-glycosylation. These glycosyltransferases are expressed in B cells and plasma cells, which are the primary producers of IgM antibodies.
In patients with severe COVID-19, there was a marked increase in the deposition of complement proteins on IgM antibodies. This suggests that the altered IgM N-glycosylation plays a role in activating the complement system, which is known to enhance the immune response. Interestingly, the study found that this increase in complement deposition could be modulated by exogenous glycosidases, enzymes that break down sugar molecules, indicating that IgM glycosylation plays a crucial role in the activation of the complement system in COVID-19.
The findings suggest that changes in IgM N-glycosylation not only affect the immune response but also have implications for the progression of COVID-19. The enhanced complement activation observed in patients with severe disease could contribute to inflammation and tissue damage, further exacerbating the severity of the infection.
Clinical Implications and Potential Biomarkers
Beyond the basic science, the findings from this study also have important clinical implications. The research suggests that specific biomarkers related to IgM N-glycosylation could be used to assess the severity of COVID-19 in patients. For example, the presence of disialylation and mannose modifications on IgM could be used to identify patients at higher risk of severe disease. Additionally, the study found that several clinical markers commonly used to assess disease severity, such as D-dimer, blood urea nitrogen (BUN), creatinine, and potassium levels, were correlated with changes in IgM N-glycosylation.
These correlations suggest that IgM N-glycosylation patterns could serve as an additional diagnostic tool for clinicians, helping to identify patients who may require more intensive treatment. Furthermore, the ability to monitor changes in IgM glycosylation throughout infection could provide valuable insights into the progression of the disease and the effectiveness of treatment strategies.
In addition, the study contributes to a broader understanding of how immune responses, particularly antibody glycosylation, can influence the outcome of viral infections. While much of the focus has been on IgG antibodies and their glycosylation patterns, the role of IgM has been less studied. This research highlights the importance of considering the full spectrum of immune responses, including IgM, when investigating the immune mechanisms underlying COVID-19.
Comparison of IgM and IgG N-Glycosylation in COVID-19
Another interesting aspect of the study is the comparison of IgM and IgG N-glycosylation patterns in patients with varying severities of COVID-19. The researchers found that IgM and IgG antibodies exhibited different N-glycosylation profiles depending on the severity of the disease. Specifically, while IgM N-glycosylation was associated with the initial immune response and complement activation, IgG N-glycosylation appeared to play a role in the later stages of the immune response.
This differential glycosylation of IgM and IgG highlights the complex nature of the immune response to SARS-CoV-2 and suggests that both antibodies may contribute to disease progression in distinct ways. Understanding how these antibodies interact with each other and with other components of the immune system could provide valuable information for developing targeted therapies and vaccines.
Conclusion
The study conducted by Comunale’s team represents a significant advancement in our understanding of the immune response to COVID-19. It demonstrates that alterations in IgM N-glycosylation are closely linked to the severity of the disease and the activation of the complement system. These findings open up new avenues for research into biomarkers for disease severity and potential therapeutic targets.
IgM N-glycosylation may serve as an important diagnostic tool for assessing the immune response in COVID-19 patients, providing clinicians with valuable information to guide treatment decisions. Moreover, the study underscores the importance of considering the full spectrum of immune responses, including both IgM and IgG, when studying the immune mechanisms of COVID-19. As research into the virus and its effects on the immune system continues to evolve, these insights may lead to more effective strategies for managing and treating COVID-19, ultimately improving patient outcomes.
Creative Biolabs is committed to advancing IgM research and development to help identify more effective therapeutic candidates. We offer a comprehensive range of services covering the entire IgM process, from discovery to production and characterization. In addition to our specialized IgM products, we also provide a variety of other offerings, including IgA and IgE products, tailored to meet the diverse needs of your research. Whether you’re looking for cutting-edge IgM solutions or exploring other immunoglobulin products, our team is dedicated to supporting your scientific endeavors with expertise and precision.
Reference
- Haslund-Gourley, Benjamin S., et al. “IgM N-glycosylation correlates with COVID-19 severity and rate of complement deposition.” Nature communications 15.1 (2024): 404. Distributed under the Open Access license CC BY 4.0, without modification.