Immunoglobulin M (IgM) is the most ancient and structurally distinctive immunoglobulin isotype in the vertebrate immune system. Though often overshadowed by IgG and IgA, IgM plays a critical role in shaping early immune responses, maintaining mucosal homeostasis, and modulating immune-related diseases. Recent advances have deepened our understanding of IgM’s structural versatility, memory capacity, and therapeutic relevance, reinvigorating interest in this unique antibody class.
The Foundations: IgM Structure and Early Expression
IgM is the first antibody isotype expressed during B cell development. It is secreted either as a pentamer or, less commonly, a hexamer, resulting in a molecule with a molecular mass exceeding 900 kDa. The pentameric structure is stabilized by a 15-kDa joining (J) chain, which facilitates high-avidity multivalent binding despite low monomeric affinity. This makes IgM an exceptional tool for agglutination, immune complex formation, and complement activation.
Two forms of IgM exist: membrane-bound IgM (mIgM) acts as a B cell receptor (BCR), while secreted IgM (sIgM) is involved in systemic and mucosal immunity. Notably, the secretory form is heavily glycosylated, a feature that regulates its bioavailability and interaction with receptors such as the polymeric immunoglobulin receptor (pIgR) and FcμR.
Distinct Pathways: IgM-Producing B Cell Lineages
IgM is produced by both B1 and B2 cell lineages. B1 cells, which originate during fetal development and populate the peritoneal and pleural cavities, secrete natural IgM antibodies that exhibit broad polyreactivity toward self and microbial antigens. These cells are considered innate-like and can act without T cell help.
B2 cells, the conventional bone marrow-derived B cells, are responsible for antigen-specific adaptive responses. After encountering antigens, B2 cells either differentiate into short-lived plasma cells (SLPCs) producing low-affinity IgM or enter germinal centers (GCs) for somatic hypermutation (SHM) and class switch recombination (CSR). However, some B2-derived plasma cells retain the IgM isotype and develop into long-lived plasma cells (LLPCs), particularly in the spleen, highlighting the lasting role of IgM in immunity.
IgM Memory: Challenging the Traditional Paradigm
One of the most compelling recent findings is the identification of IgM memory B cells (MBCs-M). These cells exhibit limited SHM compared to IgG memory B cells but persist long-term and can rapidly differentiate into antibody-secreting cells (ASCs) upon antigen re-exposure. MBCs-M contribute to recall responses independent of class switching and offer broad protection through cross-reactivity, especially against glycan-rich microbial epitopes.
In humans, MBCs-M are found in mucosal tissues such as the gut-associated lymphoid tissue (GALT), the spleen, and even peripheral blood. Their capacity to recognize conserved glycans makes them potent contributors to antibacterial, antiviral, and antifungal defenses. Their abundance in secondary lymphoid organs suggests they play a central role in both local and systemic immunity.
IgM in Disease: Protective and Pathological Roles
Autoimmunity
In systemic lupus erythematosus (SLE), natural IgM antibodies against double-stranded DNA (dsDNA) have been shown to be protective. sIgM-deficient mice crossed with lupus-prone strains develop more severe disease, which can be ameliorated by passive administration of IgM. These results emphasize a regulatory role of IgM in limiting autoimmune progression.
Cancer
Natural IgMs target tumor-associated carbohydrate antigens (TACAs), such as MUC1 and PAM-1. These antibodies may function in early tumor surveillance by activating complement-mediated lysis. Importantly, tumor-derived IgM antibodies have shown promise in diagnosis and prognosis, especially in breast cancer.
Allergy and Asthma
Although less studied, IgM deficiency is associated with allergic diseases. IgM antibodies targeting bacterial GlcNAc residues can cross-react with fungal allergens and suppress airway inflammation. These findings imply that natural IgM may participate in allergen neutralization and immune tolerance at mucosal surfaces.
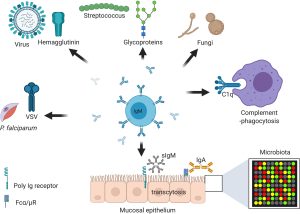
Fig.1 Immunoglobulin M (IgM) is central at steady stage and against infections and non-communicable diseases.1,2
IgM at the Mucosal Frontier
At mucosal sites, particularly in the gut and respiratory tract, IgM collaborates with IgA to regulate microbial composition. Secretory IgM binds to both commensal and pathogenic microbes, facilitating their clearance or containment. Studies show that individuals with high IgM levels harbor microbiota enriched in beneficial bacteria like Bacteroides vulgatus and Lachnospiraceae.
Moreover, IgM plays a crucial role in protecting against bacterial pneumonia. In mouse models, B1 cell-derived sIgM significantly improves survival following E. coli and S. pneumoniae infections, and this protection depends on GM-CSF and IL-1β-mediated signaling pathways. The antibody also appears critical in limiting systemic dissemination of pathogens by forming immune complexes and triggering early cytokine responses.
IgM in Pathogen Defense: From Fungi to Parasites and Viruses
Fungal Infections
Natural IgM antibodies against β-glucans and chitin components of fungal cell walls help control infections by Cryptococcus neoformans and Pneumocystis carinii. These antibodies enhance macrophage activation, promote Th1 responses, and contribute to fungal containment in the lungs and brain.
Parasitic Infections
In Trypanosoma evansi infection, IgM antibodies provide protection independently of classical inflammatory pathways like IFN-γ or TNF-α. Passive transfer of IgM—but not IgG—confers immunity. Similarly, in malaria, anti-α-gal IgM antibodies generated in the gut protect against Plasmodium infection by targeting conserved glycan motifs on the parasite surface.
Viral Infections
IgM is essential in the early control of viruses such as influenza, vesicular stomatitis virus (VSV), and respiratory syncytial virus. These antibodies facilitate viral neutralization, opsonization, and clearance via classical complement pathways. In IgM-deficient models, delayed virus clearance and increased mortality are consistently observed.
Final Thoughts: A Case for IgM in Therapeutic and Research Innovation
Despite decades of focus on IgG, IgM is proving to be more than an immunological relic. Its rapid production, glycan specificity, mucosal function, and protective versatility position it as a valuable target for diagnostics, therapeutics, and vaccine development. As scientific interest in non-IgG isotypes rises, so does the demand for specialized IgM research tools and development services.
Discover IgM Research Solutions at Creative Biolabs
Whether you’re developing IgM-based therapeutics or investigating mucosal immunity, Creative Biolabs offers tailored services to meet your needs:
-
Therapeutic IgM Antibody Discovery: Identify high-avidity IgM candidates for immune intervention.
-
Chimeric IgM Antibody Engineering: Customize IgM scaffolds for enhanced functionality.
-
IgM Production and Purification: Efficient expression and large-scale purification solutions.
-
IgM Glycosylation Analysis: Characterize critical glycan profiles for bioactivity and stability.
Ready to explore the next frontier in antibody research? Connect with our experts and harness the untapped power of IgM today.
Reference:
1. Jones, Katelyn, et al. “Immunoglobulin M in health and diseases: how far have we come and what next?.” Frontiers in immunology 11 (2020): 595535.
2.Distributed under the Open Access license CC BY 4.0, without modification.