Antimicrobial resistance (AMR) is steadily eroding the impact of conventional antibiotics. Every year, hundreds of thousands of deaths are already attributed to drug-resistant infections, and projections indicate that, without new strategies, this number could rise to millions of deaths annually by 2050, alongside a severe economic burden. In this landscape, antibody-based interventions are attracting renewed interest as targeted, resistance-sparing tools.
Among these, avian egg yolk antibodies, known as IgY, stand out as a scalable, safe, and versatile platform that can be harnessed against a wide range of bacterial, viral, and toxin targets – including multidrug-resistant (MDR) pathogens. The reviewed literature positions IgY not just as a niche alternative, but as a serious contender in the toolbox to reduce antibiotic pressure and support more sustainable anti-infective strategies.
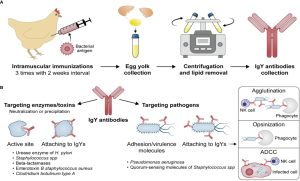
Fig.1 Development of the IgY antibodies (A) and their potential mechanisms of action (B).1
Antibiotic Resistance and the Need for New Anti-Infective Strategies
The rise of AMR is tightly linked to decades of intensive antibiotic use in human and veterinary medicine, agriculture, and food production. Pathogens have developed an array of resistance mechanisms, including:
-
Enzymatic degradation of antibiotics (e.g., β-lactamases)
-
Efflux pumps that expel drugs from bacterial cells
-
Target modification or protection, making antibiotics less effective
-
Biofilm formation that shields microbial communities from antimicrobials
Developing new antibiotics is expensive, slow, and often followed by rapid emergence of resistance. As a result, researchers are actively exploring complementary or alternative strategies, such as:
-
Vaccines to prevent infections
-
Bacteriophages and phage-derived enzymes
-
Plant-derived antimicrobial compounds
-
Monoclonal antibodies and other biologicals
IgY antibodies sit at the intersection of these approaches: they are biologics, can be directed against highly specific targets, and can be produced at scale using an animal-friendly, egg-based system.
What Are IgY Antibodies and How Are They Produced?
IgY is the major serum immunoglobulin in birds, reptiles, and amphibians, functionally analogous to mammalian IgG but structurally distinct. In laying hens, IgY is actively transported from serum into the egg yolk to protect the developing embryo. This natural transfer process is exploited for antibody production.
Typical IgY production workflow includes:
-
Immunization of hens with a defined antigen (whole cell, recombinant protein, peptide, toxin, enzyme, etc.).
-
Sustained antibody response, with high titers of antigen-specific IgY circulating in serum and accumulating in egg yolks for months.
-
Collection of eggs instead of blood, greatly improving animal welfare and enabling continuous, non-terminal harvesting.
-
Extraction and purification of IgY from yolk using precipitation, ultrafiltration, chromatographic or other scalable processes.
A single hen can yield over 20 g of total IgY per year, with a significant proportion being antigen-specific. This corresponds roughly to the yearly IgG yield from several rabbits, but without repeated blood collection or sacrifice. In practice, hens become small “antibody factories,” offering a flexible and cost-effective production system for research-grade polyclonal antibodies.
Structural and Functional Features Distinguishing IgY from IgG
Although functionally similar, IgY and mammalian IgG differ in several important aspects:
Molecular structure
-
-
IgY lacks the classic hinge region seen in IgG and instead has an extended heavy chain with four constant domains.
-
The absence of a hinge makes IgY more rigid, which can increase structural stability under certain conditions.
-
Fc-mediated interactions
-
-
IgY does not bind mammalian Fc receptors and does not activate the mammalian complement system in the same way as IgG.
-
This reduces the risk of uncontrolled inflammatory responses and minimizes antibody-dependent adverse effects when used in mammalian systems.
-
Biochemical properties
-
-
IgY is relatively resistant to trypsin and chymotrypsin, allowing partial survival in the intestinal environment.
-
It is rich in sialic acid, a feature associated with extended half-life and favorable physicochemical behavior in some contexts.
-
These features underlie the good safety profile of IgY in oral applications and support its use as a passive immunization tool in both human and veterinary research settings.
Safety and Routes of Administration for IgY
The reviewed studies highlight a strong safety record for IgY, particularly with oral use. Oral IgY has been recognized as Generally Recognized as Safe (GRAS) by regulatory authorities for certain applications, and human studies using IgY-containing preparations report good tolerability.
Evaluated administration routes include:
-
Oral administration
-
As powders, capsules, or functional foods (e.g., yogurt, lozenges, chewing tablets).
-
Mainly used to target gastrointestinal pathogens such as Helicobacter pylori, Salmonella, Campylobacter, and diarrheal viruses.
-
-
Topical or mucosal routes
-
Nasal sprays, mouthwashes, or gargles for respiratory or oral pathogens.
-
-
Inhalation / respiratory use (preclinical/clinical research)
-
For Pseudomonas aeruginosa in cystic fibrosis or chronic lung disease models, where IgY can interfere with bacterial colonization in the airways.
-
Because IgY does not engage mammalian Fc receptors or complement pathways, systemic immune activation is limited. Most reported effects are local—neutralizing toxins, blocking adherence, or aggregating pathogens—rather than triggering systemic inflammation.
Why IgY Is Attractive Compared with Conventional Antibiotics
The paper emphasizes several advantages of IgY over traditional antibiotics in the context of AMR:
Multiepitope targeting
-
-
Polyclonal IgY preparations recognize multiple epitopes on the same pathogen.
-
To escape such antibody pressure, bacteria would need simultaneous mutations in multiple genes, which is far less likely than acquiring resistance to a single antibiotic molecule.
-
Targeting virulence rather than viability
-
-
IgY often neutralizes toxins, adhesins, enzymes, or other virulence factors instead of directly killing the pathogen.
-
This “disarming” approach reduces selective pressure for survival and can limit the emergence of resistant clones.
-
Preservation of commensal microbiota
-
-
Antigen-specific IgY can focus on a defined pathogen without broadly eradicating the normal flora, in contrast to many broad-spectrum antibiotics.
-
Flexible combination strategies
-
-
IgY can be combined with existing antibiotics or acid-suppressive drugs (e.g., in H. pylori models) to enhance efficacy or shorten treatment duration.
-
Such combinations may allow lower antibiotic doses or reduced treatment cycles.
-
Sustainability and up-scaling potential
-
-
Poultry farming is highly scalable worldwide, and IgY production can be integrated into existing egg production chains.
-
This opens a path to large-volume, cost-effective antibody preparations particularly attractive for low- and middle-income regions.
-
Mechanisms of IgY Action Against Pathogens
Across multiple models, IgY antibodies exert their protective effects through several recurring mechanisms:
Blocking adhesion and colonization
-
-
IgY specific for bacterial adhesins or surface antigens can prevent attachment to epithelial cells in the gut, lungs, or oral cavity.
-
This is documented for P. aeruginosa in respiratory epithelium, Salmonella and Campylobacter on intestinal cells, and H. pylori on gastric mucosa.
-
Neutralizing toxins and enzymes
-
-
IgY raised against bacterial toxins (e.g., staphylococcal enterotoxin B, botulinum neurotoxin, cholera toxin) can neutralize their activity in vitro and in animal models.
-
IgY can also target enzymes like urease in H. pylori or β-lactamases that degrade antibiotics, thus protecting drugs or host tissues.
-
Agglutination and enhanced clearance
-
-
By coating bacterial surfaces, IgY can cause visible agglutination, reducing viable counts (CFU) and facilitating mechanical or immune clearance.
-
Even without classical Fc-mediated opsonization, IgY can promote phagocytosis in some systems, potentially via alternative receptors or non-receptor-mediated processes.
-
Modulating mucosal immunity and microbiota balance
-
-
In some animal studies, IgY administration led to changes in mucosal immune markers and microbial community composition, suggesting broader ecosystem effects beyond direct neutralization.
-
Selected Applications of IgY Against Multidrug-Resistant Pathogens
Helicobacter pylori and Gastric Pathology
H. pylori infection is a major cause of chronic gastritis, peptic ulcers, and gastric cancer risk, with treatment complicated by rising antibiotic resistance. IgY has been directed against several H. pylori antigens, including:
-
Urease subunits (e.g., UreC)
-
Adhesion-related proteins
-
Virulence factors such as CagA, NAP, and OipA
In preclinical models, anti-H. pylori IgY has been shown to:
-
Reduce bacterial colonization in the stomach
-
Decrease gastritis scores and mucosal damage
-
Attenuate urease activity and associated inflammation
Human volunteer studies using IgY-enriched egg preparations reported reductions in urea breath test values and improvements in symptom scores, especially when IgY was combined with acid-suppressive therapy. Although complete eradication was not consistently achieved, the data support the concept of IgY as a supportive tool to manage drug-resistant H. pylori in research and development settings.
Pseudomonas aeruginosa in Respiratory Infections
P. aeruginosa is a key driver of chronic lung infections in cystic fibrosis and other chronic lung diseases, often exhibiting multidrug resistance. Anti-Pseudomonas IgY has been evaluated in vitro and in animal models, demonstrating:
-
Inhibition of bacterial adhesion to the oropharynx
-
Reduction in colonization and load in respiratory tissues
-
Enhanced phagocytic uptake by polymorphonuclear neutrophils despite lack of classical Fc-receptor engagement
These findings suggest that IgY targeting P. aeruginosa could be explored as a non-antibiotic strategy to reduce bacterial burden and protect vulnerable patient populations in preclinical research.
Enteric Pathogens: Salmonella and Campylobacter
In poultry and livestock, Salmonella and Campylobacter are major zoonotic pathogens and important reservoirs for resistant strains. IgY generated against these bacteria has been tested in chicks and other animal models where:
-
Oral administration of target-specific IgY reduced bacterial counts in the cecum.
-
Colonization and fecal shedding were diminished, especially when IgY was combined with probiotics.
-
Adhesion to intestinal epithelial cells (e.g., Caco-2) was blocked, limiting early colonization events.
Such data underpin the idea that IgY could be integrated into feed additives or functional products to support control of enteric pathogens in the food chain, thereby indirectly contributing to reduced antibiotic use.
Acinetobacter baumannii and Other MDR Bacteria
Acinetobacter baumannii is a notorious nosocomial pathogen with extensive resistance to multiple drug classes. The review describes IgY generated against outer membrane proteins such as OmpA and Omp34, which:
-
Inhibit the growth of pan-drug-resistant strains in a dose-dependent manner
-
Reduce bacterial adhesion to host cells
-
Induce structural changes on the bacterial surface that compromise colonization
These examples, together with IgY against Staphylococcus aureus, Vibrio cholerae, and others, illustrate the breadth of bacterial targets that can be addressed by the IgY platform.
Viral Targets and Toxin Neutralization
Beyond bacteria, IgY has been raised against a variety of viral antigens, including influenza, rotavirus, dengue, and Zika viruses. In lethal challenge models, virus-specific IgY has provided protection without inducing antibody-dependent enhancement, a critical safety issue in some flavivirus infections.
IgY directed against toxins (e.g., staphylococcal enterotoxin B, botulinum toxin) and cholera toxin further demonstrates its potential in neutralizing secreted virulence factors that drive disease severity.
Challenges and Future Directions for IgY Technology
Despite its promise, the IgY field still faces several challenges before wider adoption in regulated products:
Standardization of production
-
-
Differences in bird strains, immunization protocols, and extraction methods can introduce batch-to-batch variability.
-
Harmonized guidelines and quality frameworks are needed for more consistent, GMP-like production where required.
-
Formulation and delivery
-
-
IgY is sensitive to low pH and pepsin in the stomach; microencapsulation, enteric coatings, and protective matrices are under active investigation to preserve activity after oral administration.
-
Regulatory and licensing pathways
-
-
While IgY-based nutraceuticals and functional foods exist in some markets, clear regulatory routes for more advanced IgY-based products are still developing.
-
Expanded target space and engineering
-
-
Combining IgY with modern antibody engineering, recombinant expression, or fusion designs could unlock new formats and functional profiles.
-
Overall, the review concludes that IgY antibodies are well-positioned to complement antibiotics and other biologics in the fight against MDR pathogens, especially where passive immunization and local neutralization strategies are desired.
How Creative Biolabs Supports IgY Antibody Research
To help researchers translate these concepts into practical solutions, Creative Biolabs provides a complete suite of IgY-related services on the Non-IgG Antibody Platform:
These services are designed to support IgY-based projects from concept through to advanced preclinical research, enabling scientists to efficiently explore egg-derived antibodies as a powerful alternative in the era of antibiotic resistance.
Reference:
1.El-Kafrawy, Sherif A., et al. “IgY antibodies: The promising potential to overcome antibiotic resistance.” Frontiers in immunology 14 (2023): 1065353. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1021/acs.jmedchem.4c01257