HIF-1α: Orchestrating Metabolic Rewiring for IgA Synthesis
The hypoxic microenvironment of germinal centers (GCs) is a critical niche where B cells undergo clonal expansion and antibody diversification. Central to this process is hypoxia-inducible factor 1α (HIF-1α), a transcription factor that reprograms cellular metabolism to meet the energetic demands of antibody production. Studies reveal that B cells lacking HIF-1α exhibit impaired glycolytic flux, a metabolic shift essential for sustaining high-energy processes like immunoglobulin class-switch recombination (CSR). Specifically, HIF-1α deficiency disrupts acetyl-CoA accumulation, a metabolite pivotal for histone acetylation and transcriptional activation of IgA switch regions (Sα).
In experimental models, B cells with HIF-1α deletion fail to generate adequate IgA levels, exacerbating dextran sodium sulfate (DSS)-induced colitis. Conversely, stabilizing HIF-1α via prolyl hydroxylase (PHD) inhibitors enhances IgA CSR and mitigates intestinal inflammation. These findings underscore HIF-1α’s dual role: it acts as both a metabolic rheostat and a gatekeeper of epigenetic modifications necessary for IgA synthesis.
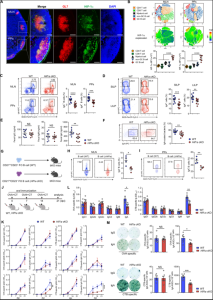
Fig.1 Loss of HIF-1α in B cells leads to impaired IgA-producing cell differentiation.1
- Acetyl-CoA: Bridging Metabolism and Epigenetic Regulation
The interplay between metabolism and epigenetics is exemplified by acetyl-CoA, a key metabolite in the HIF-1α-driven pathway. Acetyl-CoA serves as a substrate for histone acetyltransferases (HATs), enabling histone H3K27 acetylation at the Sα locus. This epigenetic modification loosens chromatin structure, facilitating access for activation-induced cytidine deaminase (AID) and subsequent CSR to IgA.
Notably, HIF-1α-deficient B cells exhibit reduced acetyl-CoA levels, impairing H3K27 acetylation and IgA production. Rescue experiments with acetyl-CoA supplementation restore IgA synthesis and ameliorate colitis in these models. This metabolic-epigenetic crosstalk highlights how cellular fuel availability directly shapes antibody diversity. Moreover, it positions acetyl-CoA as a therapeutic target for disorders characterized by IgA deficiency.
- IgA Dynamics in Gut Homeostasis and Inflammation
IgA dominates mucosal immunity, coating commensal bacteria to prevent dysbiosis and neutralize pathogens. Its production is tightly regulated by local signals, including TGF-β, IL-5, and IL-6, which synergize with HIF-1α to promote CSR to IgA. In the gut, IgA-secreting plasma cells depend on hypoxic niches, where HIF-1α sustains their metabolic fitness and antibody output.
Disruptions in this axis have profound consequences. For instance, DSS-induced colitis models demonstrate that HIF-1α-deficient mice suffer from severe intestinal damage due to insufficient IgA-mediated microbial containment. Conversely, boosting HIF-1α activity enhances IgA coating of pathobionts, reducing inflammation and preserving epithelial integrity. These observations align with clinical data linking the IgA deficiency to inflammatory bowel disease (IBD) susceptibility.
- PHD Inhibitors: Harnessing Hypoxia Signaling for Therapy
Pharmacological stabilization of HIF-1α via PHD inhibitors, such as roxadustat, offers a promising strategy to enhance IgA production and combat gut inflammation. By mimicking hypoxia, these inhibitors amplify HIF-1α-driven glycolysis and acetyl-CoA synthesis, thereby restoring epigenetic modifications required for IgA CSR. Preclinical studies show that PHD inhibitors not only increase IgA levels but also reduce pro-inflammatory cytokines like TNF-α and IL-6 in colitis models.
Beyond IgA modulation, PHD inhibitors exert broader immunomodulatory effects. For example, they promote regulatory T cell (Treg) differentiation and suppress Th17-mediated inflammation, further mitigating gut pathology. However, long-term safety and tissue-specific effects remain under investigation, particularly regarding unintended oncogenic risks associated with sustained HIF-1α activation.
- The Gut Microbiome: A Key Player in IgA Regulation
The gut microbiome actively shapes IgA repertoires through Toll-like receptor (TLR) signaling and metabolite production. Commensal bacteria like Bacteroides thetaiotaomicron induce T cell-independent IgA CSR via polysaccharide antigens, while segmented filamentous bacteria (SFB) drive T cell-dependent responses. HIF-1α intersects with these pathways by modulating B cell responsiveness to microbial signals. For instance, butyrate—a bacterial metabolite—enhances HIF-1α stability, linking microbial metabolism to host antibody production.
In dysbiosis, reduced IgA coating permits pathogenic overgrowth, triggering inflammation. Therapeutic interventions, such as probiotics engineered to produce lactate (e.g., EcNLac), can activate HIF-1α-NDUFA4L2 signaling in dendritic cells (DCs), indirectly bolstering IgA responses and restoring microbial balance.
Future Directions: Precision Targeting of the HIF-1α Pathway
Emerging technologies, including single-cell sequencing and CRISPR-based screens, are unraveling the tissue-specific roles of HIF-1α in immunity. For example, gut-restricted PHD inhibitors aim to minimize systemic side effects while maximizing local IgA production. Similarly, engineered IgA monoclonal antibodies (e.g., W27) show promise in selectively neutralizing pathobionts without disrupting commensals, offering a tailored approach for IBD.
Furthermore, combining HIF-1α modulators with existing therapies—such as anti-TNF agents or microbiota transplants—could synergize to reset immune homeostasis. Challenges remain in balancing pro- and anti-inflammatory outcomes, but the metabolic-epigenetic axis centered on HIF-1α represents a frontier for next-generation immunotherapies.
Conclusion
The discovery of HIF-1α’s role in IgA synthesis underscores the intricate link between cellular metabolism, epigenetic regulation, and mucosal immunity. By reprogramming B cell energetics and chromatin landscapes, HIF-1α ensures robust IgA production, a cornerstone of gut health. Therapeutic strategies targeting this axis, from PHD inhibitors to microbiome engineering, hold transformative potential for treating inflammatory disorders. As research advances, precision modulation of HIF-1α pathways may unlock novel avenues to restore immune balance and combat diseases rooted in IgA dysregulation.
In the dynamic biopharmaceutical landscape, non-IgG antibody therapeutics have emerged as a transformative force, offering unprecedented opportunities for targeted treatment modalities. At Creative Biolabs, we stand at the vanguard of this scientific revolution, integrating profound scientific acumen with an expansive array of innovative platforms to empower every stage of therapeutic innovation – from conceptual validation to clinical translation.
Our multidisciplinary team orchestrates a symphony of advanced technologies including:
- High-throughput functional screening systems
- Species-specific isotype characterization tools
- Customizable bispecific/multispecific formats
Initiate your discovery acceleration by scheduling a technical consultation with our antibody architects. Explore how our differentiated platform can de-risk your development pipeline and unlock novel biological space.
Recommended Services
| Services | Features | Price |
| Therapeutic IgA Antibody Discovery
|
|
Inquiry |
| Chimeric IgA Antibody Engineering
|
· Specialized in chimeric IgA antibody production with recombinant DNA technology.
|
Inquiry |
| IgA Production and Purification |
|
Inquiry |
| IgA Glycosylation Service
|
|
Inquiry |
Reference:
- Meng, Xianyi, et al. “Metabolic rewiring controlled by HIF-1α tunes IgA-producing B-cell differentiation and intestinal inflammation.” Cellular & Molecular Immunology1 (2025): 54-67. Distributed under the Open Access license CC BY 4.0, without modification.