Cancer immunotherapy continues to revolutionize treatment paradigms, yet advanced melanoma remains a challenging battlefield. Despite notable progress with immune checkpoint inhibitors like anti-PD-1 and CTLA-4 antibodies, long-term survival is still elusive for a significant subset of patients. This unmet need has spurred interest in alternative immunotherapeutic modalities, including one unexpected yet promising player: the IgE antibody.
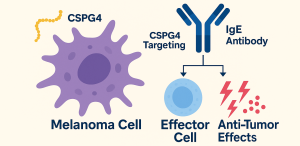
In a groundbreaking approach, researchers have harnessed IgE’s potent immune-activating properties to develop a new class of antibody therapy targeting chondroitin sulfate proteoglycan 4 (CSPG4), a melanoma-associated antigen. This strategy not only addresses critical gaps in current melanoma treatments but also opens the door to a new generation of non-IgG antibody therapeutics.
CSPG4: A Prime Target in Melanoma
CSPG4 is a membrane-bound proteoglycan overexpressed in approximately 70% of melanomas, including metastatic and treatment-resistant forms, while exhibiting minimal presence in healthy tissues. Transcriptomic analyses confirm markedly elevated CSPG4 mRNA levels in melanoma compared to other cancers or normal skin, and immunohistochemical studies have demonstrated robust expression in 63% of tumor samples, both in primary and metastatic sites.
Functionally, CSPG4 contributes to tumor cell adhesion, migration, and invasion—critical processes for melanoma progression. Its consistent and high expression in tumor tissues, combined with low levels in normal counterparts, makes it an ideal antigen for targeted therapy.
Why IgE? Rethinking the Antibody Toolbox
Most antibody-based therapies use the IgG isotype, which is well-characterized and widely accepted. However, IgE presents unique biological advantages that are particularly compelling for oncology:
-
High affinity for FcεRI receptors found on monocytes, macrophages, and basophils
-
Long tissue residency, enabling sustained local immune engagement
-
Potent pro-inflammatory potential, which can reshape the tumor microenvironment
Capitalizing on these traits, researchers engineered a chimeric IgE antibody specific to CSPG4, aiming to channel IgE’s immune-activating features directly toward tumor eradication.
Constructing the CSPG4-IgE Antibody: Design and Validation
The development process involved combining murine-derived variable regions targeting CSPG4 with human IgE constant domains. Structural assessments using SDS-PAGE and size-exclusion chromatography confirmed the antibody’s integrity and purity (>95%).
Functionally, the antibody bound selectively to CSPG4-positive melanoma cell lines (A2058, A375) and human tumor tissues, with negligible interaction with CSPG4-negative cancers or healthy samples. Importantly, there was no cross-reactivity with murine CSPG4, ensuring preclinical model reliability.
Engaging the Immune System: IgE-Mediated Mechanisms
1. ADCC via Monocyte Activation
In co-culture experiments, the CSPG4-IgE antibody induced robust antibody-dependent cellular cytotoxicity (ADCC) against CSPG4-expressing melanoma cells. Peripheral blood mononuclear cells (PBMCs) from both healthy donors and melanoma patients demonstrated significant cytolytic activity. Monocytes were identified as the primary effectors, and their removal abrogated the ADCC response.
2. Pro-Inflammatory Reprogramming
The antibody also activated innate immune pathways. Upon crosslinking CSPG4-IgE on monocytes, researchers observed upregulation of inflammatory cytokines such as TNF, IL-1β, IL-6, and CCL2/MCP-1. This was accompanied by elevated expression of co-stimulatory molecules (CD80, CD86) and a reduction in immunosuppressive markers (CD163), indicating a shift toward an anti-tumor phenotype.
3. Macrophage Recruitment and Infiltration
In vivo, CSPG4-IgE treatment enhanced tumor infiltration by CD68+ macrophages. Transcriptomic analysis revealed enrichment in gene signatures associated with monocyte/macrophage activity and pro-inflammatory cytokine responses, including TNF, IFN-γ, and IL-12 signaling.
Demonstrated Anti-Tumor Efficacy in Multiple Models
Subcutaneous Melanoma Xenografts
In immunodeficient mice reconstituted with human PBMCs, CSPG4-IgE significantly suppressed tumor growth compared to isotype controls and CSPG4-IgG. Notably, the antitumor effect persisted with infrequent dosing, suggesting strong local tissue retention despite rapid systemic clearance.
Lung Metastasis Models
When administered intravenously, CSPG4-IgE reduced the metastatic burden in the lungs, indicating systemic activity against disseminated melanoma.
Patient-Derived Xenografts (PDX)
In highly relevant PDX models using autologous melanoma tissues and immune cells, CSPG4-IgE prolonged survival, underscoring its translational potential. Mechanistically, tumor transcriptomes showed activation of FcεRI signaling, antigen presentation, and interferon pathways.
Addressing Safety Concerns: Low Risk of Allergic Responses
Given IgE’s role in allergic reactions, safety remains a primary concern. However, the researchers conducted rigorous tests to assess hypersensitivity risk. Basophil activation tests using patient blood showed no activation by CSPG4-IgE, even at high doses. Likewise, sera from healthy donors and patients failed to induce degranulation in RBL cells.
These findings align with prior studies indicating that IgE antibodies targeting tumor-restricted antigens are unlikely to cause systemic hypersensitivity due to the absence of crosslinking antigens in circulation.
Redefining the Therapeutic Landscape for Melanoma
The emergence of CSPG4-IgE introduces a new class of antibody therapy that may overcome current limitations in melanoma immunotherapy. Key benefits include:
-
Precision targeting: CSPG4 specificity minimizes damage to healthy tissues.
-
Innate and adaptive immune synergy: Activation of monocytes and macrophages bridges the immune response spectrum.
-
Broad potential: Effective across metastatic, primary, and PDX tumor models.
This approach could be particularly impactful when used alongside checkpoint inhibitors or adoptive T cell therapies. Moreover, CSPG4 is expressed in other malignancies, such as breast cancer and mesothelioma, hinting at broader oncological applications.
Service Spotlight: Empowering Your IgE Research
At Creative Biolabs, we’re committed to pioneering the future of antibody therapeutics. Our extensive expertise in non-IgG antibody development—especially IgE—equips researchers with the resources to translate promising concepts into viable therapies.
| Service | Key Features |
|---|---|
| Therapeutic IgE Antibody Discovery | Innovative discovery platforms, advanced characterization techniques, and tailored antibody design |
| Chimeric IgE Antibody Engineering | Humanized constant domains for immune compatibility, suitable for various disease models |
| IgE Antibody Production and Purification | High-yield production and scalable purification for preclinical and clinical research |
| IgE Glycosylation Service | Detailed glycan profiling and modification strategies to optimize therapeutic function |
Our solutions span from early antibody screening to late-stage production, enabling seamless integration into your development pipeline.
Final Thoughts: Charting a New Course in Oncology
CSPG4-IgE represents a paradigm shift in how we think about antibody-based cancer therapy. By moving beyond the conventional IgG model and embracing the distinct immunobiology of IgE, this strategy offers a two-pronged attack—direct tumor targeting and immune modulation.
As researchers and biotech innovators explore the vast potential of non-IgG formats, Creative Biolabs stands ready to support these endeavors with scientifically grounded, end-to-end services. Contact us today to bring your IgE-based therapeutic vision to life.