Allergic diseases share a common inflammatory logic: epithelial “danger” signals activate a type-2 cascade that culminates in IgE-armed effector cells and rapid symptom flares on re-exposure. Monoclonal antibodies (mAbs) that intercept this cascade at three control points—IgE, type-2 cytokines, and epithelial alarmins—have reshaped care across asthma, chronic spontaneous urticaria (CSU), atopic dermatitis (AD), chronic rhinosinusitis with nasal polyps (CRSwNP), eosinophilic esophagitis (EoE), and food allergy. This article distills the key mechanisms, clinical signals, and practical selection principles to help translational teams and clinical innovators navigate the expanding toolbox.
From Barrier Injury to IgE Memory: the Allergic Cascade in Brief
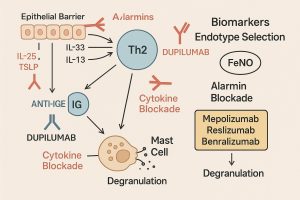
Barrier damage in skin, airways, or gut triggers epithelial release of alarmins—TSLP, IL-25, and IL-33—which prime dendritic cells, activate group-2 innate lymphoid cells (ILC2s), and drive Th2 polarization. Th2 outputs (IL-4, IL-5, IL-13) promote B-cell class switching to IgE, recruit eosinophils, and remodel tissue. IgE then arms mast cells and basophils via FcεRI; upon allergen re-exposure, these cells degranulate within minutes, producing clinical symptoms.
Two mechanistic details matter for therapy design:
-
Redundancy upstream: The three alarmins signal through distinct receptor systems yet converge on similar downstream type-2 programs—one reason multi-node control can outperform single-node control in some settings.
-
Persistence downstream: IgE binds FcεRI with uniquely slow off-rates, conferring long-lived “antigen memory” to tissue mast cells even when circulating IgE declines—an important consideration for sequencing and duration of anti-IgE therapy.
The IgE Axis: Direct and Indirect Strategies
Direct Anti-IgE
Neutralizing circulating IgE prevents its loading onto FcεRI and can gradually reduce effector cell sensitivity. Clinical experience supports anti-IgE across multiple indications, particularly in IgE-driven endotypes; assessing total and allergen-specific IgE helps identify likely responders, though extremely high IgE (e.g., in some AD cohorts) may predict weaker responses.
Beyond classical anti-IgE antibodies, engineered IgE scavengers are emerging. In early clinical evaluation, an IgE-binding “trap” showed dose-proportional exposure and deeper, longer suppression of free serum IgE than first-generation agents, with only mild to moderate adverse events—supporting further trials in atopic conditions.
Targeting IgE Regulation (CD23)
CD23 negatively regulates IgE synthesis. Preclinical and early clinical data with anti-CD23 antibodies demonstrated decreased serum IgE, while mechanistic studies show CD23 engagement can reduce IgE production and eosinophilic infiltration. These findings highlight B-cell and regulatory checkpoints upstream of IgE that could complement direct neutralization.
Takeaway: Direct IgE neutralization remains the most validated IgE-centric approach, while CD23 and engineered traps broaden upstream control options—potentially useful when FcεRI-bearing cells maintain reactivity despite modest IgE reductions.
Type-2 Cytokine Blockade: Tailoring by Biology and Biomarkers
IL-4/IL-13 via IL-4Rα (Dupilumab)
Dupilumab blocks IL-4Rα, thereby inhibiting both IL-4 and IL-13 pathways. This dual action explains its broad approvals (AD, asthma, CRSwNP, EoE, prurigo nodularis, and CSU in certain regions) and multi-node impact on type-2 inflammation. Importantly, dupilumab can prevent IgE class switching in vitro and reduce IgE over time in vivo, situating it upstream of anti-IgE therapies. Clinically meaningful differences vs anti-IgE have not been established in head-to-head studies; biomarker-guided selection (e.g., elevated eosinophils or high FeNO) is recommended.
Mechanistic nuance matters: IL-4 is essential for IgE class switching, whereas IL-13 is more involved in tissue effects (fibrosis, goblet cell hyperplasia). This explains why IL-4Rα blockade can influence both IgE biology and structural disease aspects.
IL-5/IL-5R (Mepolizumab, Reslizumab, Benralizumab)
IL-5 is the central survival and recruitment cue for eosinophils. Three agents target this axis with different modalities:
-
Mepolizumab (anti-IL-5) reduces eosinophils and exacerbations in severe eosinophilic asthma and is also used in EGPA, HES, and as add-on for CRSwNP.
-
Reslizumab (anti-IL-5) shows high in-vitro affinity; clinical outcomes are broadly similar to mepolizumab when populations are matched.
-
Benralizumab (anti-IL-5Rα) adds ADCC-mediated eosinophil apoptosis, effectively reducing exacerbations and improving lung function in severe eosinophilic asthma.
This family is phenotype-specific: benefits concentrate in eosinophilic endotypes rather than across all allergic diseases.
Alarmin Blockade: Upstream Control at the Epithelial Interface
Tezepelumab is a human IgG2 mAb that neutralizes the epithelial alarmin TSLP. By cutting the earliest “danger-signal” link between barrier tissue and type-2 immunity, tezepelumab improves lung function and reduces exacerbations regardless of baseline eosinophils—and lowers FeNO and IgE, implying broader network suppression (likely through dampened IL-5/IL-13 outputs and lower IL-4/IL-13-driven class switching). Early data in CSU are promising, suggesting utility beyond asthma where alarmin-dominated biology is active.
More generally, TSLP, IL-25, and IL-33 are released by epithelial and barrier-adjacent cells in response to stress and damage. Although each signals through distinct receptors, their overlapping outputs argue for careful patient selection—and, in certain contexts, for multi-node strategies when single-node blockade underperforms.
Which mAb for Which Patient? Practical Selection Signals
A growing menu of biologics enables endotype-guided and preference-sensitive care—but it also raises the bar for diagnostics and shared decision-making. Guidance frameworks emphasize aligning mechanism with biomarkers (e.g., baseline eosinophils, FeNO, total/specific IgE), comorbidities, and practical aspects (dose, route, storage, patient lifestyle). An at-a-glance view of approved mAbs by indication underscores the point: no single agent dominates every allergic setting.
Signals that often guide choice:
-
IgE-driven patterns (clear IgE sensitization, higher total/sIgE): consider anti-IgE or IL-4Rα (the latter with additional tissue benefits). Note that very high IgE (certain AD cohorts) may blunt anti-IgE responses.
-
Eosinophilic airway inflammation (blood eosinophils, frequent exacerbations): prioritize IL-5/IL-5R or, if broader phenotype or mixed biomarkers, TSLP.
-
Barrier-proximal activation (high FeNO, multi-trigger asthma, upstream epithelial activation): TSLP inhibition offers endotype-agnostic exacerbation control and multi-marker improvements.
What about combinations? Dual-mAb case reports (e.g., anti-IgE plus anti-IL-5) exist for severe, refractory asthma, but robust prospective evidence is still limited; selection should be individualized with careful safety and value assessment.
Emerging Targets and Future Directions
Upstream circuit breakers (alarmins) and downstream memory nodes (FcεRI-bound IgE) define the “bookends” of type-2 control. Future gains may come from:
-
Smarter IgE control: high-capacity traps, IgE-specific depletion strategies, and optimized sequencing with IL-4Rα blockade to disrupt class-switch inputs while unloading FcεRI over time.
-
Context-aware alarmin targeting: choosing TSLP/IL-33/IL-25 strategies by organ system and remodeling biology (e.g., asthma versus AD) rather than disease labels alone.
-
Sharper endotyping: integrating IgE kinetics, FeNO, eosinophils, and clinical triggers to match mAbs to the most actionable drivers for each patient.
Key Takeaways
-
Multiple therapeutic gates exist in allergy—IgE, type-2 cytokines, and alarmins—and each gate corresponds to distinct patient biology and biomarker profiles.
-
Dupilumab acts upstream of IgE, slowing class switching and lowering IgE over long horizons, while anti-IgE rapidly unloads FcεRI to blunt immediate reactivity. Use biomarkers and endotypes to prioritize.
-
IL-5/IL-5R therapies excel in eosinophilic airway disease; TSLP blockade offers broad exacerbation control and multi-marker improvements, even when eosinophils are low.
Related Services at Creative Biolabs (Non-IgG Antibodies)
Looking to translate these insights into projects focused on IgE—from discovery through analytics and manufacturing? Our Non-IgG Antibody team supports end-to-end IgE workflows:
-
Therapeutic IgE Antibody Discovery — lead identification and optimization for IgE formats, FcεRI engagement, and antigen specificity.
-
IgE Glycosylation Service — site-specific characterization and engineering to tune stability, effector interactions, and function.
-
IgE Production & Purification — scalable expression and purification solutions tailored to IgE structure.
-
IgE Antibody Products — browse our catalog of IgE antibodies and related tools for research and development.
Reference:
1. Eggel, Alexander, Luke F. Pennington, and Theodore S. Jardetzky. “Therapeutic monoclonal antibodies in allergy: targeting IgE, cytokine, and alarmin pathways.” Immunological Reviews 328.1 (2024): 387-411. https://doi.org/10.1111/imr.13380