Immunoglobulin M (IgM) is a crucial component of the primary immune response. As the first line of defense, these antibodies are known for their high avidity—the collective strength of multiple antibody-antigen binding sites—which compensates for the typically lower affinity of their individual binding sites. IgM molecules are produced in two main polymeric forms: hexamers, composed of six μ2L2 subunits, and pentamers, which contain five μ2L2 subunits and a single protein known as the J-chain (JC). While both forms are effective in the systemic immune response, the presence of the J-chain is what enables pentameric IgM to bind to polymeric Ig receptors (pIgR) and be transported across epithelial cells, a process called transcytosis. This unique function is vital for mucosal immunity, making the formation of a pentameric structure an important biological process.
For a long time, the molecular mechanism that dictates whether an IgM molecule forms a hexamer or a J-chain-containing pentamer remained a mystery. A recent study by Giannone et al. sheds light on this process, revealing a remarkable mechanism where the J-chain actively outcompetes a sixth IgM subunit during assembly. This blog post will delve into the key findings of this groundbreaking research, explaining the role of the J-chain’s unique properties, the importance of specific hydrophobic interactions, and the critical function of the quality control factor ERp44 in ensuring the correct assembly and secretion of these vital antibodies.
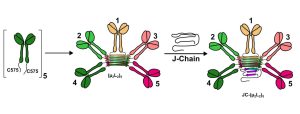 Fig.1 Assembly of pentameric IgM.1,2
Fig.1 Assembly of pentameric IgM.1,2
The J-Chain’s Unstructured Nature is Key to its Success
The study discovered that before it is inserted into an IgM molecule, the J-chain exists as a largely unstructured and highly flexible polypeptide. It is also highly susceptible to proteases and contains a variety of non-native, or heterogeneous, disulfide bonds. This disordered state is not a sign of a flawed protein; rather, it is a key feature that allows the small, flexible J-chain to fit into the tight space available in a growing IgM pentamer, giving it a crucial advantage over the bulkier, more rigid μ2L2 subunit.
This “delayed folding” mechanism means that the J-chain is essentially “ready” to fill the final spot in the pentameric structure, whereas a sixth μ2L2 subunit is slower to assemble. The researchers demonstrated this using both in cellula and in vitro experiments. They found that when co-expressed with IgM subunits, the J-chain prevented the formation of hexamers and led to the creation of pentamers. Similarly, in a test tube, adding J-chain to a reaction forming IgM oligomers caused a dramatic shift from hexamers to pentamers, confirming that the J-chain is responsible for directing this assembly.
The flexibility of the J-chain also explains its appearance in experiments. Under non-reducing conditions, the protein appears as a smeared, diffuse band, a pattern consistent with a multitude of isoforms containing different arrangements of intra-chain disulfide bonds. This smear collapses into a single, sharp band when reducing agents are added, further confirming its flexible, multi-isoform nature.
The Critical Role of Hydrophobic Interactions and Disulfide Rearrangements
The study proposes a step-by-step model for pentamer assembly, starting with the formation of the five-subunit core. At this stage, the nascent pentamer exposes hydrophobic residues on its tailpieces that are uniquely arranged to accommodate the J-chain. This creates an “encounter complex” based on hydrophobic interactions between the J-chain and the IgM core.
Specific hydrophobic residues in the J-chain—I40, F61, and Y63—were identified as essential for this initial binding. Mutating these residues to alanine or serine significantly impaired J-chain insertion and led to a failure to prevent hexamer formation. This was particularly evident with the I40A mutation, which completely prevented the hexamer-to-pentamer shift. The F61A and Y63A mutations also caused issues, leading to the formation of polymers that were poorly secreted by cells, suggesting they were not correctly assembled.
Once the initial hydrophobic contact is made, the J-chain is triggered to undergo a series of crucial disulfide rearrangements. The J-chain’s C15 and C69 residues form covalent bonds with the C575 residues on the IgM subunits. The C13-C15 pair was found to be particularly important for initiating this process, with mutations to these residues severely inhibiting pentamer formation. This disulfide reshuffling and covalent bonding ultimately stabilize the J-chain-containing pentamer and trigger its folding into a final, stable conformation.
ERp44: The Gatekeeper of IgM Quality Control
The assembly process is not without its challenges. The study found that cells lacking the quality control factor ERp44 secrete a continuous smear of J-chain molecules with heterogeneous, non-native disulfide bonds. This indicates that ERp44 plays a crucial role as a “gatekeeper,” surveying IgM assembly and preventing the secretion of improperly assembled or aberrant conformers.
The study found that ERp44 binds to the J-chain via its C29 residue, and this interaction is key to preventing its secretion. When ERp44 is overexpressed, it restores J-chain retention and promotes the formation of covalent complexes. This ensures that only correctly formed IgM polymers—whether hexamers or pentamers—are secreted, maintaining the integrity and effectiveness of the immune system.
Conclusion: A Precise and Coordinated Mechanism
The work by Giannone et al. provides a comprehensive and elegant model for how the immune system produces two distinct forms of high-avidity IgM, each with a specific function. The process is a beautifully choreographed sequence of events: a small, flexible J-chain, rich in non-native disulfide bonds, outmaneuvers a larger IgM subunit to fill the final spot in a growing pentamer. This is facilitated by a precise “fit” based on key hydrophobic interactions, which in turn triggers a cascade of disulfide rearrangements that lead to covalent stabilization and final folding. All of this is overseen by the quality control factor ERp44, ensuring that only the correctly assembled antibodies are released for either systemic or mucosal immunity.
This research highlights the profound efficiency and sophistication of the biological machinery behind the immune system, revealing how even a seemingly simple choice—pentamer or hexamer—is governed by a complex and highly regulated molecular dance.
Partner with Creative Biolabs for Your IgM Antibody Research
At Creative Biolabs, we specialize in the discovery, engineering, and production of IgM antibodies for therapeutic and research applications. Based on the mechanisms revealed in recent research, our platforms can help you harness the unique potential of IgM.
We offer:
With extensive expertise in non-IgG antibody platforms, Creative Biolabs is your trusted partner for advancing IgM-based therapeutics from concept to reality.
Reference:
1. Giannone, Chiara, et al. “How J-chain ensures the assembly of immunoglobulin IgM pentamers.” The EMBO Journal 44.2 (2025): 505-533.
2.Distributed under the Open Access license CC BY 4.0, without modification.