Allergic diseases, affecting over 300 million people globally, are characterized by dysregulated immune responses to harmless antigens. Central to this pathology is the high-affinity IgE receptor (FcεRI), a multimeric complex that orchestrates mast cell activation and the release of inflammatory mediators. Recent research highlights the pivotal role of the FcεRIβ subunit in regulating receptor trafficking, signaling, and its potential as a therapeutic target. This blog explores the structural and functional intricacies of FcεRIβ, its splicing variants, and emerging strategies to modulate allergic inflammation.
FcεRI Structure and Function: The Role of FcεRIβ
The high-affinity IgE receptor, FcεRI, exists in two isoforms that dictate its cellular specificity and function. In mast cells and basophils, the receptor predominantly adopts a tetrameric structure (αβγ₂), composed of an IgE-binding α subunit (FcεRIα), a β subunit (FcεRIβ), and a dimeric γ subunit (FcεRIγ) responsible for intracellular signaling. The β subunit, encoded by the MS4A2 gene, acts as a critical scaffold during receptor assembly. It facilitates proper folding and glycosylation of the α subunit in the endoplasmic reticulum (ER) and stabilizes interactions with the γ subunit dimer, ensuring efficient trafficking of the mature receptor complex to the cell surface. In contrast, immune cells such as monocytes and dendritic cells express a trimeric form (αγ₂) lacking FcεRIβ, which results in reduced receptor stability and altered signaling dynamics.
Beyond its structural role, FcεRIβ amplifies IgE-mediated signaling pathways. Upon antigen-induced crosslinking of IgE-bound receptors, the β subunit enhances signal transduction by stabilizing the receptor complex at the plasma membrane, delaying internalization and degradation. Importantly, FcεRIβ contains an immunoreceptor tyrosine-based activation motif (ITAM) that recruits and activates the kinase Syk, initiating downstream cascades such as the MAPK and NF-κB pathways. These events drive mast cell degranulation, lipid mediator synthesis, and cytokine production, which collectively underpin the early and late phases of allergic inflammation. Thus, FcεRIβ serves as both a molecular chaperone for receptor assembly and a signaling amplifier, positioning it as a central regulator of mast cell hyperactivity in allergic disease.
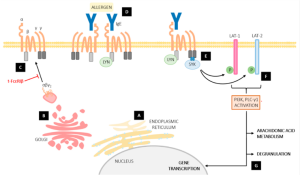
Fig.1 The role of FcεRIβ in mast cell signaling pathways.1
FcεRIβ Splicing Variants: A Double-Edged Sword
The MS4A2 gene undergoes alternative splicing to generate truncated FcεRIβ isoforms with opposing functional roles. One major variant, FcεRIβT (MS4A2 variant 2), retains intron 5, introducing a premature stop codon that eliminates the third and fourth transmembrane domains and the C-terminal immunoreceptor tyrosine-based activation motif (ITAM). Another isoform, t-FcεRIβ (MS4A2 variant 3), skips exon 3, resulting in the loss of the first two transmembrane domains. This cytoplasmic/nuclear membrane-localized variant cannot interact with FcεRIα, disrupting its integration into the receptor complex. These splicing events are dynamically regulated by cellular signals, such as IL-4 or IgE crosslinking, which modulate spliceosome recruitment to MS4A2 pre-mRNA.
While full-length FcεRIβ promotes receptor stability and signaling, its truncated counterparts act as competitive inhibitors. FcεRIβT binds FcεRIα in the endoplasmic reticulum but redirects it to proteasomal degradation instead of enabling γ subunit assembly, reducing surface FcεRI expression by up to 80%. Paradoxically, this loss of surface receptors amplifies allergic responses in vivo. Murine studies reveal that FcεRIβT overexpression triggers compensatory IgE overproduction and sensitizes mast cells to low-dose antigens, exacerbating hypersensitivity. Similarly, t-FcεRIβ disrupts receptor trafficking and sequesters signaling intermediates, yet may independently regulate nuclear pathways linked to mast cell survival. Thus, while splicing diversifies FcεRIβ function, imbalances in isoform ratios tilt the balance toward pathological hypersensitivity, underscoring its dual role as both modulator and instigator of allergic inflammation.
Targeting FcεRIβ: Therapeutic Opportunities
Current therapeutic strategies against IgE-mediated allergic inflammation primarily focus on neutralizing IgE (e.g., omalizumab) or disrupting FcεRIα signaling. However, these approaches face limitations: omalizumab fails to dissociate preformed IgE-FcεRI complexes and requires burdensome weight- and IgE level-based dosing, while FcγRIIb co-engagement strategies risk off-target immunosuppression by broadly inhibiting Fc receptor signaling across immune cell types. These challenges highlight the unmet need for mast cell-specific therapies that directly target FcεRIβ—a subunit selectively expressed in mast cells and basophils—to minimize systemic side effects.
Emerging approaches aim to exploit FcεRIβ’s unique regulatory roles. Splice-switching antisense oligonucleotides (SSOs) designed to promote nonfunctional FcεRIβ isoforms, such as t-FcεRIβ, show promise in preclinical models, reducing surface FcεRI expression and mast cell degranulation in a dose-dependent manner. Similarly, small-molecule inhibitors targeting FcεRIβ-associated kinases (e.g., Syk) or mast cell survival pathways (e.g., KIT) are under investigation, though their lack of cell specificity remains a hurdle. Advances in tissue-targeted delivery systems, such as lipid nanoparticles conjugated to mast cell-specific antibodies, could enhance the precision of these therapies. By selectively modulating FcεRIβ trafficking or splicing, these strategies offer a path to dampen allergic hyperactivity without compromising broader immune functions.
Unmet Needs and Future Directions
Despite advances in understanding FcεRIβ biology, significant unmet needs persist, including underdiagnosis of allergic disorders and the complexity of heterogeneous multi-organ syndromes, such as the asthma-rhinitis-eczema triad, which complicate therapeutic strategies. Future efforts must prioritize precision approaches that account for tissue-specific differences in mast cell behavior; for example, lung and skin mast cells exhibit divergent FcεRIβ splicing patterns, necessitating tailored delivery systems to optimize targeting. Additionally, combination therapies—such as pairing splice-switching oligonucleotides with anti-IgE biologics—could synergistically dampen inflammation while minimizing compensatory pathways or resistance. Further exploration of FcεRIβ’s regulatory roles in non-allergic contexts and its interaction with other immune receptors may also unveil novel therapeutic avenues. Addressing these challenges will require interdisciplinary collaboration to bridge gaps between mechanistic insights, diagnostic tools, and clinically viable interventions.
Conclusion
FcεRIβ is a linchpin in allergic inflammation, governing receptor trafficking, signaling amplification, and mast cell responsiveness. Its splicing variants add layers of complexity, offering both challenges and therapeutic avenues. While antisense oligonucleotides and splice-switching therapies hold promise, addressing diagnostic gaps and tissue-specific heterogeneity will be critical for next-generation interventions. As research unravels the nuances of FcεRIβ biology, the dream of precision allergy medicine inches closer to reality.
Creative Biolabs has assembled an experienced team of scientists who have been studying non-IgG antibody research for decades, dedicated to providing Therapeutic IgE Antibody Discovery solutions to clients worldwide. In addition, we can provide a full range of IgE antibodies from different species, such as rats, mice, humans, and goats for different applications. For more detailed information, please feel free to contact us or send us an inquiry.
Reference:
Arthur, Greer K., and Glenn Cruse. “Regulation of trafficking and signaling of the high affinity IgE receptor by FcεRIβ and the potential impact of FcεRIβ splicing in allergic inflammation.” International Journal of Molecular Sciences 23.2 (2022): 788. Distributed under the Open Access license CC BY 4.0, without modification.