Non-IgG antibodies—especially IgA, IgM, and IgE—are re-entering biologics pipelines for reasons IgG cannot always match: mucosal targeting, high avidity through multivalency, and specialized effector biology. But the same structural features that make these isotypes exciting also make them harder to manufacture, characterize, and stabilize. In practice, many non-IgG programs don’t fail on target engagement; they fail when the molecule meets real-world CMC constraints: aggregation, product heterogeneity, and stability loss during expression, purification, fill–finish, or storage.
This article breaks down why these three developability risks show up early in non-IgG projects, what “good” looks like for controlling them, and how to build a decision-ready developability strategy before you commit to expensive scale-up.
Why Non-IgG Developability Is a Different Game
With IgG, the industry has decades of playbooks: established expression systems, platform purification, and familiar analytical comparability expectations. Non-IgG formats add layers of complexity that directly increase the probability of early CMC derailment:
-
Higher-order assembly and valency
IgM commonly forms pentamers (often with a J chain) or hexamers; IgA can be monomeric or polymeric; these assemblies introduce more interfaces where mispairing and aggregation can occur. -
Heavier and more diverse glycosylation
Non-IgGs can carry more N-glycans (and, in some contexts, additional O-glycosylation), expanding the heterogeneity space and altering thermal/colloidal behavior. -
More disulfide and domain architecture variability
Additional interchain linkages, alternative tailpieces, and isotype-specific constant-region features can create sensitivity to redox conditions, pH shifts, and shear. -
Less “platform” process compatibility
Even if the binding domain behaves nicely, the isotype scaffold may require custom expression tuning, non-standard purification logic, and a more deliberate formulation strategy.
The implication is simple: developability must be treated as a front-loaded design variable, not a downstream QC checkbox.
Aggregation: The First (and Loudest) Signal of Trouble
Aggregation is often the earliest visible symptom of a non-IgG developability problem because it can be triggered by multiple stressors—temperature excursions, concentration increases, pH transitions, freeze–thaw cycles, mixing, filtration, and even routine hold steps.
What makes non-IgGs aggregation-prone?
Aggregation risk tends to rise when a molecule has (1) more interaction surfaces and (2) more opportunities for partial unfolding:
-
Multimer interfaces and assembly defects (notably IgM and polymeric IgA) can create populations that are “almost right,” yet unstable.
-
Hydrophobic patches on variable domains or exposed constant-region areas can drive self-association, especially at high concentration.
-
Glycan-dependent conformational effects may stabilize one region while destabilizing another, producing a delicate balance that shifts with process conditions.
-
Mechanical sensitivity (shear, agitation, pumping) can be more pronounced for large, multimeric structures.
How aggregation shows up in real projects
You might see:
-
Increased high molecular weight (HMW) species after a buffer exchange
-
SEC profiles that drift with hold time
-
Unexpected losses during sterile filtration (adsorption + aggregation)
-
Lot-to-lot variability tied to subtle upstream differences (temperature, feed strategy, harvest timing)
A practical control mindset for aggregation
A robust approach typically combines engineering + process + formulation, rather than betting on a single fix:
-
Molecule-level mitigation
-
Reduce surface hydrophobicity or known self-association motifs via targeted substitutions
-
Revisit domain orientation, linker choices, or constant-region variants if the format allows
-
Evaluate glycoengineering approaches when specific glycoforms correlate with aggregation
-
-
Process-level mitigation
-
Optimize capture and polishing to remove aggregation seeds early
-
Tighten redox/pH control during steps that affect disulfide integrity and folding
-
Minimize high-shear handling and design gentle mixing/transfer strategies
-
-
Formulation-level mitigation
-
Screen pH and ionic strength for colloidal stability (not just solubility)
-
Use excipients strategically (stabilizers, surfactants, tonicity agents) based on stress pathways
-
Run agitation and freeze–thaw stress early—non-IgGs often reveal weaknesses here faster than IgG
-
Aggregation is not just a quality attribute; it is a trajectory. If the aggregation slope is steep at small scale, it rarely improves by accident at larger scale.
Heterogeneity: When “One Antibody” Is Actually a Population
Non-IgG heterogeneity can be broader than many teams anticipate. And when heterogeneity is poorly understood, it becomes difficult to define specifications, comparability strategies, or even reliable potency correlations.
Common heterogeneity sources in non-IgG formats
-
Glycoform diversity
More glycosylation sites can mean more combinations of occupancy and structure (fucosylation, sialylation, branching, etc.), affecting charge, stability, and receptor interaction. -
Size variants
-
Misassembled multimers (especially in IgM and polymeric IgA)
-
Fragmentation or partial clipping
-
Low levels of aggregates that expand over time or under stress
-
-
Charge variants
Deamidation, oxidation, C-terminal processing, glycan sialylation differences, and other micro-heterogeneities can generate complex icIEF patterns and shift over storage. -
Sequence- and process-linked variants
Translation errors are rare but upstream conditions can modulate folding quality, disulfide formation, and glycan processing—creating variability that looks like “mystery peaks” unless investigated systematically.
The analytics stack that reduces ambiguity
For non-IgG developability, analytics should be selected to answer three practical questions:
-
What variants exist?
Use orthogonal methods (e.g., SEC for size, CE-SDS for purity/fragmentation, icIEF for charge, LC–MS for mass and glycosylation). -
Which variants matter?
Connect variants to function with fit-for-purpose assays (binding, cell-based activity, receptor engagement where relevant), and evaluate which attributes correlate with performance. -
Can we control them?
Establish whether the dominant drivers are sequence-intrinsic, expression-host related, or purification/formulation related—and then implement a control strategy that scales.
A common mistake is treating heterogeneity as “just characterization.” In reality, heterogeneity is often the map that tells you where your process is fragile and where your molecule needs reinforcement.
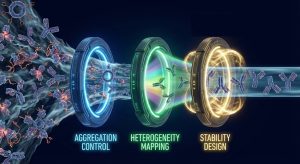
Stability: Thermal, Chemical, and Mechanical—All at Once
Stability is not a single property. For developability decisions, it’s useful to separate stability into three interacting layers:
1) Conformational (thermal) stability
This is your molecule’s resistance to unfolding and structural rearrangement. Tools like DSC or nanoDSF can reveal transitions that predict vulnerability during temperature excursions or extended holds.
Non-IgG reality: large multimeric formats may show multiple transitions, and a “stable” domain can still coexist with a weak interface that drives aggregation later.
2) Chemical stability
Oxidation, deamidation, disulfide scrambling, glycan changes, and other modifications can accumulate during storage or under stress. These can shift charge variants, reduce potency, or create new degradation pathways.
Non-IgG reality: additional glycans and disulfide complexity can expand the chemical stability landscape—meaning you need early forced-degradation insight to avoid surprises.
3) Colloidal and mechanical stability
This is about self-association, viscosity, sensitivity to agitation, and filterability at the concentrations you actually need.
Non-IgG reality: high valency and large hydrodynamic size can increase viscosity or promote reversible self-association that later becomes irreversible aggregation under stress.
Building a stability strategy that’s decision-ready
Instead of running “generic stability,” structure your early work to produce actionable outcomes:
-
Define real use conditions (target concentration, container, shipping profile, freeze–thaw expectations)
-
Stress test with intent (agitation, thermal ramps, pH transitions, light exposure if relevant)
-
Track both structure and function (analytics + potency/binding readouts)
-
Select a formulation window based on the dominant degradation pathway, not on habit
When stability is approached this way, you’re not just collecting data—you’re reducing program risk with each experiment.
Putting It Together: A Developability Playbook for Non-IgG Programs
A practical non-IgG developability workflow often looks like this:
-
Early screening (multiple candidates, fast filters)
Identify obvious red flags: high baseline HMW, unstable icIEF patterns, poor thermal profile, agitation sensitivity. -
Focused characterization (top candidates)
Map heterogeneity drivers, confirm assembly quality, and evaluate manufacturability behaviors (capture robustness, hold stability, filterability). -
Mitigation loop (engineering + process + formulation)
Apply targeted improvements and re-test under stress conditions that mirror production reality. -
Lock a control strategy for scale-up
Define critical quality attributes (CQAs), critical process parameters (CPPs), and comparability-ready assays early—this is where non-IgG programs win time.
The goal is not to make a molecule “perfect.” The goal is to make it predictable, controllable, and scalable.
Recommended Creative Biolabs Services for Non-IgG Developability
If you’re advancing an IgA/IgM/IgE program and want to reduce aggregation risk, clarify heterogeneity, and strengthen stability early, these pages are a good starting point: